| CAS
NO. |
8030-78-2 |
|
| EINECS NO. |
232-447-4 |
| FORMULA |
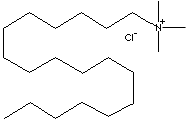
CH3(CH2)17N(Cl)(CH3)3 |
| MOL
WT. |
|
|
H.S.
CODE
|
2923.90 |
|
TOXICITY
|
|
| SYNONYMS |
Tallowtrimonium Chloride;
|
| Quaternary ammonium compounds, trimethyltallow alkyl,
chlorides; Trimetilsebo alquil, cloruros (Spanish); Triméthylsuif alkyles, chlorures
(French); |
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
(30% AQ. SOLUTION)
|
| PHYSICAL
STATE |
Clear to pale yellow liquid |
|
MELTING
POINT
|
20
C |
| BOILING
POINT |
100
C |
| SPECIFIC GRAVITY |
0.95 |
|
SOLUBILITY
IN WATER
|
Soluble |
| pH |
6
- 9 (10% Aq. solution)
|
| VISCOSITY |
|
|
AUTOIGNITION |
|
|
REFRACTIVE
INDEX
|
|
| NFPA RATINGS |
Health: 2; Flammability: 0; Reactivity: 0 |
| FLASH
POINT |
|
| STABILITY |
Stable under ordinary
conditions |
|
APPLICATIONS
|
Quaternary ammonium compounds are any of a group of ammonium salts in which
organic radicals have been substituted for all four hydrogens of the original
ammonium cation. They has a central nitrogen atom which is joined to four
organic radicals and one acid radical. The organic radicals may be alkyl, aryl,
or aralkyl, and the nitrogen can be part of a ring system. They are prepared by
treatment of an amine with an alkylating agent. They show a variety of physical,
chemical, and biological properties and most compounds are soluble in water and
strong electrolytes. In addition to their tendency of locating at the interface
of two phases (liquid–liquid or solid–liquid) to introduces continuity between
the two different phase, they have properties of disrupting micro-organisms'
cell processes. These compounds are used as ;
- Surface-active
agents
- Solvents
- Intermediates
- Active Ingredient for Conditioners
- Antistatic Agent
- Detergent Sanitisers
- Softner for textiles and paper
products
- Phase Transfer Catalyst
- Antimicrobials
- Disinfection Agents
And Sanitizers
- Slimicidal Agents
- Algaecide
- Emulsifying Agents
- Pigment Dispersers
|
| SALES
SPECIFICATION |
|
ACTIVE
MATTER
|
28.0%
min
|
|
HLB
|
21
|
|
pH
|
6
- 9 (10% Aq. solution)
|
|
COLOR,
GARDNER
|
3
max
|
|
CARBON
CHAIN
|
C18 65% + C16
30% + C14 5% + C12 1% |
| TRANSPORTATION |
| PACKING |
180kgs
in Drum |
| HAZARD CLASS |
8
(Packing Group: III) |
| UN
NO. |
1759 |
|
GENERAL
DESCRIPTION OF FAT |
Tallow is a refined hard fat extracted from fatty deposits of animals,
especially from suet (fatty tissues around the kidneys of cattle and sheep).
Fats produced by organic processes in plants are palm, coconut, palm kernel,
sunflower, soybean, and other oils. Their main components are triolein and
triglyceryl esters of stearic (C18), palmitic (C16), myristic(C14), lauric
(C12), oleic (C18:1), and other fatty acids. The molecules of most natural fatty
acids have an even number of carbon chains due to the linkage together by ester
units. Analogous compounds of odd numbers carbon chain fatty acids are also
stable and can be made synthetically. All fats are insoluble in water and have
lighter weight than water. Industrial fats can be sub-classified as fat, grease
or oil depending on melting point. Fats that are liquid at room temperature are
referred to oil. Grease has a higher initial viscosity than oil. It is used as a
lubricant. The organic processes to convert fats to fatty acids (or esters)
and glycerol is called oleochemistry. Fatty acids and glycerol are produced by
hydrolysis water of the triglycerides. Fatty acid esters and are produced by
esterification reaction. Coconut or palm oils are better source to get saturated
fatty acids, while sunflower or soybean oils are good source to obtain
unsaturated fatty aids such as oleic acid (C18:1) and linoleic acid
(C18:2). Generally, commercial coconut fatty acid has carbon chain
composition of; C10: 5% max + C12: 45 - 55% + C14: 20 - 25% + C16: 10 - 15 %
+ C18: 10 - 15% max( including unsaturated fatty acids). Fats are used to
make soap, food products, cosmetics, and candles, and lubricants. They are
wisely used in producing synthetic surfactants.
|
